Equine herpesvirus-1 (EHV-1) and equine herpesvirus-4 (EHV-4) are large double-stranded DNA viruses that are ubiquitous pathogens of horses. Estimates of prevalence show most adult horses are infected with EHV-1, EHV-4, or both throughout their lifespan and establishment of lifelong latency is detected in up to 70% of infected horses. Infection with EHV-1/4 is one of the most common causes of viral respiratory disease in horses worldwide.
Clinical Signs
Clinically, the respiratory disease caused by EHV-1 and EHV-4 is indistinguishable and can be mild or asymptomatic in older or previously exposed horses. In contrast, the respiratory disease observed in young immunologically naïve horses is often severe, lasts for two to three weeks, and is characterized by a biphasic fever, depression, anorexia, coughing, and oculonasal discharge that is initially serous and then becomes mucopurulent. Further, EHV-1 causes equine herpesvirus myeloencephalopathy (EHM), late-term abortions in the last trimester of pregnancy, death of neonatal foals, and chorioretinopathy. In contrast to respiratory disease, the risk for abortions in the third trimester of pregnancy and outbreaks of neurological disease is of greater significance in middle aged or older horses. EHV-4 has been implicated occasionally in causing late term abortions and EHM, but its etiological role is considered to be very minor and this occurs far less commonly than is observed with EHV-1.
Differences in Pathogenesis
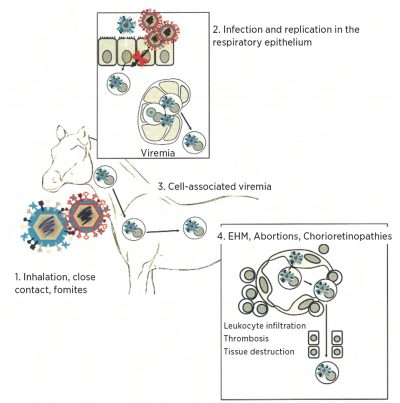
Pathogenesis of EHV-1 (virus in blue) and EHV-4 (virus in red)
- EHV-1/4 infect v 1. ia the respiratory tract.
- EHV-1/4 infect and replicate in the respiratory epithelium. EHV-1, but
not EHV-4, infects local lymphoid cells via cell-to-cell contact.
A cell-associated viremia is established for EHV-1 and transports the virus to secondary replication sites.
The vascular endothelium of the CNS , pregnant uterus or eye is infected via contact with infected lymohocytes, leading to EHM, abortions, and chorioretinopathies.
(Courtesy of Dr. Gabriele Landolt, Colorado State University)
Outbreaks of EHM
In recent years, outbreaks of EHM have increased in North America. Because of this, the Center for Emerging Issues released an emerging disease notice report regarding the neurologic form of EHV-1 in January 2007. Despite this knowledge and strong efforts to control this disease, EHM outbreaks continue to be a problem. In May of 2011 the largest outbreak ever was reported and in the first quarter of 2018 (January - March), the Equine
Disease Communication Center (www.equinediseasecc.org/) lists more than 20 outbreak alerts for confirmed or suspected cases of EHM nationwide.
The reason for this increase in the prevalence of EHM is not clear, and there are a number of identified viral, host, and environmental components that factor into the incidence of EHM. These include age, breed, gender, season, past exposure, a secondary fever several days after primary exposure, stress, magnitude and duration of viremia, and infection with the D752 genotype.
To date, the most important identified factor is the essential role of viremia in transmitting the virus to the vasculature of the CNS. Allen et al. found that EHV-1 strains with high neuropathogenic potential are characterized by a longer duration and greater magnitude of viremia when compared to EHV-1 strains with a low neuropathogenic potential. This evidence supports the argument that prolonged exposure of the CNS vascular endothelium to high viral loads increases the risk of EHM. Further, the identification of a single nucleotide polymorphism in the viral polymerase gene that results in a coding change (N752 to D752) has been associated with increased neuropathogenicity and high levels of viremia.
Diagnostic Options
Diagnosis of equine herpesvirus infections in the laboratory include methods to detect viral DNA, infectious virus, viral proteins, and antibodies.
- Deep nasal or nasopharyngeal swabs are sufficient to diagnose respiratory disease induced by EHV-1 or EHV-4.
- When it is important to assess the risk of virus transmission, abortion or EHM associated with EHV-1, a combination of deep nasal or nasopharyngeal swabs and whole blood collected in EDTA is required. Since fever is closely correlated to virus shedding of EHV-1, it is important to take temperatures of horses on infected farms twice daily and to submit samples from febrile horses. Testing of asymptomatic horses is done to verify absence of shedding at the end of the quarantine period.
- PCR is the most commonly used method to detect viral DNA in diagnostic samples and differentiate between EHV-1 and EHV-4. A positive PCR signal obtained from a nasal swab may indicate virus shedding, but is not absolute proof since non-infectious virus can be present. Positive PCR results from whole blood extracts indicate that a horse is viremic, which can lead to EHM or transplacental infection. It is recommended, therefore, that both a nasal swab and whole blood are submitted and tested separately.
- A follow-up test to a positive PCR result is to type the strain detected. This is done by a PCR assay that targets a single nucleotide polymorphism in the DNA polymerase gene. The result of this typing typically demonstrates whether a “neurotropic” or “non-neurotropic” strain has been detected and has to be interpreted in the context of the information currently available. Approximately 75% of the samples from horses with EHM are infected with the D752 genotype, which labels them as neurotropic. However, the so-called nonneurotropic (N752) strain is detected in the other 25% of EHM cases. It is clear that EHM is not exclusively associated with a single genetic marker within the viral genome.
- Virus isolation (VI) is regarded as the gold standard method, since it detects infectious virus. A positive VI result is directly correlated to active virus shedding and potential for transmission. Limitations of VI are potential loss of infectious virus during storage or mailing and the three to four day minimum turnaround time of a VI attempt.
- Immunohistochemistry is a very useful method to examine formalin fixed tissues for the presence of viral antigens. An advantage of this morphological method is that a positive test directly correlates the detection of viral proteins within specific histologic lesions.
- Virus neutralization testing is available for the retrospective diagnosis of equine herpesvirus infections. It is essential that paired sera, collected within a three to four week interval, are submitted for this testing. A titer increase that is at least four-fold between the acute and convalescent samples is needed to make the diagnosis. Standard virus neutralization tests do not distinguish between antibodies induced by EHV-1 or EHV-4.
Our laboratory has the capability to test for the presence of equine herpesviruses by all the approaches described above. In addition, type-specific neutralization tests, which differentiate between antibodies induced by EHV-1 or EHV-4, will be introduced later this year. For more information on specific collection protocol, sample and shipping requirements, and other relevant details, please see our list of available tests at animalhealth.msu.edu or contact the laboratory at 517.353.1683.
