By: VDL Clinical Pathologists (Jennifer Thomas, Michael Scott, Julia Stickle, Cheryl Swenson, Cynthia Lucidi)
Introduction
Masses or other lesions are identified via physical examination or diagnostic imaging. While these techniques provide information about the size, shape, consistency, and appearance of a lesion, additional testing is often required for a definitive diagnosis. Cytology is a non-invasive tool to collect cells for microscopic examination to help make a diagnosis, choose an appropriate therapeutic course of action, or provide prognostic information. Risks are minimal and include hemorrhage or spread of infection or neoplastic cells.
Well-made cytology smears provide excellent morphologic detail of cells and organisms and evaluation can often help differentiate general disease processes such as inflammation, hyperplasia or benign neoplasia, and malignant neoplasia. Differentiation of specific types of neoplasia may require additional techniques such as surgical biopsy with histologic examination or immunophenotyping.
Sample Collection
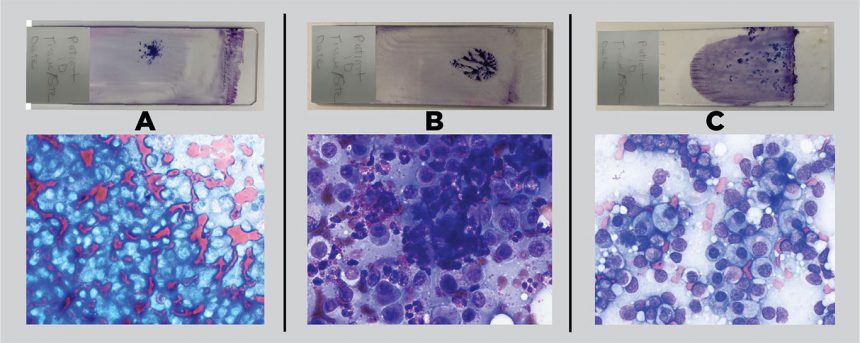
Fine needle biopsy (FNB) is commonly used to collect diagnostic cytology samples. The goal is to produce a monolayer of cells with minimal cell rupture. If the sample is not well-spread, cells cannot be adequately evaluated (figure 1). It is important to spread material in the center of the slides because material along the ends of slides cannot be evaluated under higher magnifications because stage slide holders obstruct microscope lenses in these locations.
Samples from skin or subcutaneous lesions can usually be obtained with physical restraint alone. Sedation or anesthesia may be required for specimen collection from body cavity tissues or fractious patients. Palpation of superficial lesions helps identify the features of the lesion and guide the collection process.
Collection from multiple different regions is warranted if the lesion is large or has areas with variable features (e.g., palpated density, ultrasound characteristics). Slides from different sites should be identified (e.g., site 1, site 2). A single lesion may have intermixed areas of inflammation, necrosis, neoplasia, or normal tissue cells. If a lesion contains a fluid filled center, collection from both fluid and surrounding solid regions is recommended; fluid examination alone rarely reveals the nature of the surrounding mass.
Minimal equipment is required for FNB: needle (usually 20- to 22-gauge), syringe (usually 6 to 12 mL), and clean glass slides. Larger-bore needles tend to yield more blood or thick cores of tissue that cannot be adequately spread, but sometimes may be required for poorly exfoliating lesions.
Non-aspiration and aspiration techniques can be used. Non-aspiration collection helps minimize blood contamination of preparations; the area to be sampled is immobilized and a needle (without attached syringe) is rapidly moved using sharp forward cutting motions and being careful to avoid moving beyond the tissue of interest. Redirect the needle and repeat the process 1 to 2 times to increase the likelihood of collecting a representative sample; if the mass is large enough, repeat the process in another area with a new needle. Remove the needle from the tissue and expel the material onto slides for spreading as described below.
The aspiration technique may be used if the non-aspiration technique yields poorly cellular preparations; place the needle attached to a syringe into the tissue and withdraw the plunger slightly to apply gentle negative pressure. Continue as for the non-aspiration technique with repeated forward cutting motions. Avoid excessive suction and do not leave the needle in one place and repeatedly aspirate as these approaches tend to cause hemorrhage that dilutes the sample, making evaluation more difficult. Release the negative pressure before redirecting the needle. Do not exit the mass while applying negative pressure, or material may be aspirated and lost into the syringe. With either technique, diagnostic material will often be present in the hub of the needle and not visible.
Slide Preparation
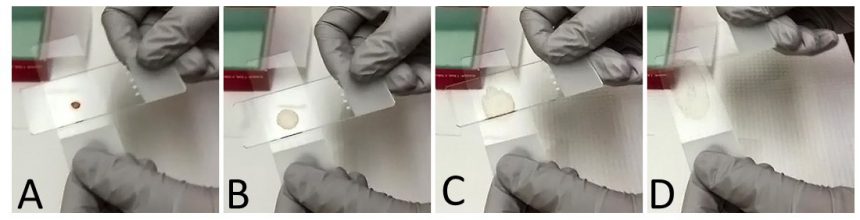
Immediately after removing the needle from the tissue, attach a syringe filled with air and rapidly expel the contents of the needle onto a glass slide by depressing the plunger. The sample may clot in the needle if this step is delayed.
Touch the needle tip to the slide with the bevel facing down to expel the material as a single drop. Spraying from a distance tends to produce small droplets that dry before the sample can be spread, resulting in thick clumps of cells that cannot be evaluated well.
Place the material in the middle 2/3 of the slide, more toward the frosted edge. Quickly but gently spread the drop of material using a blood smear wedge technique (if very liquid) or a gentle squash technique (figure 2).
For the horizontal squash technique, gently place a clean glass slide on top of the slide with the sample (gravity only). Pull the top slide along the bottom slide using a continuous motion while applying minimal pressure to spread the material.
Carefully label the slides, using a pencil, with patient information and tissue, including sites if multiple areas from a lesion are collected. Frosted slides make labeling easier. If using stickers or tape to label slides for submission to the VDL, label the slide on the opposite side of the sample so it does not interfere with our automated stainer.
Shipping and Staining
After preparation, smears should be air dried, protected (e.g., from flies, scratches, dust) in a slide holder, and stored at room temperature until staining. Heat fixation is almost never recommended because it destroys cells. Wet fixation (e.g., alcohol) is not necessary for stains commonly used in veterinary medicine.
When submitting samples to the VDL, submit unstained smears along with any smears stained in the practice. It is helpful to stain and review at least one smear to make sure there are sufficient intact cells to evaluate. If smears are not diagnostic, collect a new sample either from a different region of the lesion or using a different technique. If submitting stained smears that were evaluated using immersion oil, submit these in a separate holder to prevent oil from leaking onto unstained smears.
To prevent breakage in transit, do not ship slides in flat cardboard containers unless they are enclosed in a larger rigid box. Rigid plastic or styrofoam containers are preferred for shipping.
If shipping cytology smears with formalin-fixed tissue, protect unstained smears from formalin fumes by placing the smears and tissue in separate zip top plastic bags; include absorbent material with the formalin sample to contain any spills. Double bagging samples is best. Exposure to formalin fumes may cause poor sample staining precluding adequate evaluation of the cells and accurate cytologic interpretation.
For More Information
Visit the Clinical Pathology Section on our website at animalhealth.msu.edu to learn more about chemistry, cytology, hematology and hemostasis, and preparation of cytologic samples for immunocytochemistry. Find all the tests we offer, including turnaround time, collection protocol, specimen requirements, and submittal procedures, in the MSU VDL’s catalog of available tests.
Please do not hesitate to contact us at 517.355.1774 if you have any questions or concerns about Clinical Pathology testing. We would like to help you with sample management before the sample is collected.
