The first step of cytology is about choosing a road to travel in your pursuit of a diagnosis. But before you can choose the best route you need to have appropriate and readable data and slides, i.e., the signposts. It is NOT helpful if you have too many signs piled deep and obscuring the information of another sign. It is also NOT helpful if the signs are badly damaged and no longer readable. Making good diagnostic smears of fluid samples is the same thing as having appropriate signage for your diagnostic journey.
When a fluid sample is collected, the gross appearance can be very deceptive. Septic exudates may vary from clear to opaque. Placing your sample into an appropriately sized EDTA tube can prevent clot formation and keep the cells individualized for further enumeration, preparation of slides, and cytologic evaluation.
Nucleated cell concentrations and estimation of total protein are very helpful in understanding the causes of effusions. These data can be obtained by submitting your EDTA fluid sample to the Clinical Pathology unit of DCPAH or within your clinic. Nucleated cell concentrations can be determined manually (often using Unopette systems and hemocytometers) or through automated systems when the fluid is free of excessive debris. Total protein concentration is typically estimated using a refractometer.
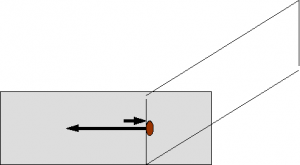
Slides should be prepared as soon as possible using the ‘blood smear’ technique (Figure 1) on both direct and concentrated (centrifuged) fluid. Blood smears can be prepared with a feathered edge or collecting cells by stopping the smear before the fluid has been distributed (stop-flow technique). Direct smears are very useful in highly cellular fluids where no concentration is needed to evaluate sufficient numbers of cells. Concentration techniques are essential for fluids with low cellularity. Smears are usually prepared from sediment produced after centrifuging the sample in a conical tipped tube (as for urine sedimentation).
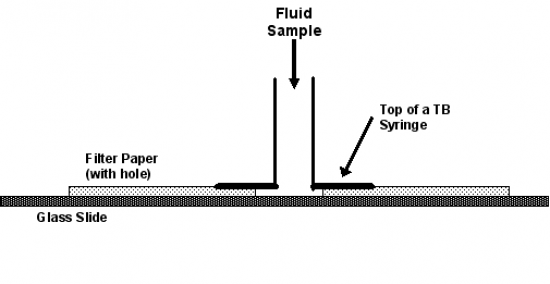
Alternatively, a simple concentration unit can be constructed (Figure 2); the cells are allowed to settle onto the glass slide while the fluid is absorbed by the filter paper.

Smears should be prepared from any particles or bits of material in a fluid sample using the two slide method (Figure 3), or by rolling the material on the slide. If larger particles are present, the rolling reduces cell smearing and breakage. The goal is to minimize cell damage and interpret intact cells that are sufficiently spaced and spread to reveal both nuclear and cytoplasmic detail. Slides should be promptly prepared, labeled (source and technique), air dried, and protected from flies, debris, chemicals, cooling, and breakage.
Slides can be stained with any good hematologic stain for inhouse review and/or submitted to the DCPAH (with the fluid) for evaluation. If fluid samples are submitted, they should be chilled (NOT frozen) but the slides should be kept at room temperature.
