The accuracy of results is only as reliable as the quality of the sample tested. Pre-analytic factors can negatively impact a sample, making it impossible for the Clinical Pathology Section to provide accurate test results that reflect any abnormalities in a patient. Some of these factors are physiologic in the patient and cannot be controlled. However, many of the sample problems that we see are avoidable and a direct result of improper sample collection, handling, or transport.
Delayed Separation of Serum from the Clot
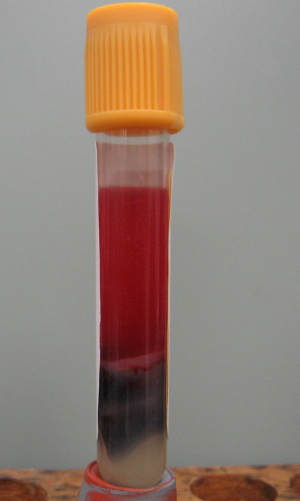
One of the most frequent problems is submission of serum for chemistry testing that has not been removed from a clot tube or is in a serum separator tube (SST). Do not rely on the gel in an SST to keep serum separate from the clot, because the gel may not seal completely or may break down in transit. This is illustrated by a sample from an adult horse (Figure 1) that arrived at the Clinical Pathology laboratory in an SST 2 days after collection. The serum had moderate hemolysis. Abnormal results on a serum chemistry profile included:
- hyperkalemia (8.2 mmol/L; reference interval 2.6-4.8)
- hyperphosphatemia (8.3 mg/dL; reference interval 5-4.7)
- hypoglycemia (<5 mg/dL; reference interval 78-124)
These abnormalities can be explained by problems with the sample. There is no way to accurately predict what the results would have been if the sample had been handled properly. Consumption of glucose by cells in the clot resulted in false hypoglycemia. While hemolysis may occur in vivo, it often occurs during sample collection, centrifugation, or shipping. The degree to which hemolysis interferes with some chemistry testing depends on the analyzer and methods used, and may occur for several reasons: 1) The red color may interfere with spectrophotometric assays. 2) Erythrocytes may release substances that positively or negatively interfere with enzyme reactions. 3) Erythrocytes contain high concentrations of some substances that will falsely elevate serum concentrations when released from the cells. In this horse, hyperkalemia and hyperphosphatemia can be explained by leakage from erythrocytes. Hyperkalemia is not expected with hemolyzed samples from cats or dogs, except for some Akitas and Shiba Inus.
Tips to prevent hemolysis include:
- avoid excess negative pressure during blood collection
- for tubes with clot activators, gently mix (do not shake) blood following collection
- avoid heating or freezing whole blood samples
- employ proper centrifugation
- separate serum from the clot promptly after collection
Aged Samples
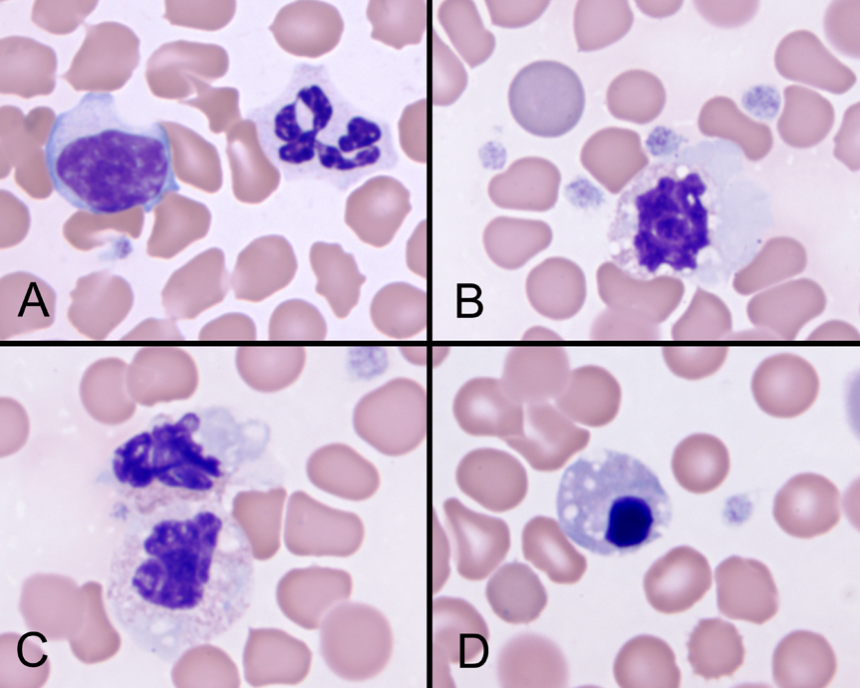
Another common pre-analytic problem is submission of aged blood or other body fluids for analysis. Cells deteriorate in vitro. Deterioration may make it impossible to identify cell features or even cell types on cytologic and hematologic preparations. Figure 2 is an image of a blood smear that was prepared from 7-day-old blood that arrived in the laboratory. Deterioration of the leukocytes made it impossible to provide a WBC differential.
To maximize information from blood or body fluids:
- submit 2 unstained, air-dried slides prepared from a gently-mixed sample (see Fluid Cytology Preparations from the Fall 2008 issue for tips on making slides of body fluids)
- ship the smears in a plastic holder to protect from breakage
- insulate the slides from the ice pack and fluid
- submit the EDTA tube with an ice pack, making sure that the fluid is insulated from direct contact with the ice pack to avoid freezing
- ship overnight to minimize transport time
Visit the DCPAH website (www.animalhealth.msu.edu) for other tips to avoid pre-analytic errors. You may also contact the Clinical Pathology Section directly at 517.355.1774 if you have questions about sample collection or handling.
