Vector-borne illnesses, those spread by insects and ticks, are on the rise according to a recent report from the Centers for Disease Control and Prevention (CDC). For equine practitioners and horse owners, two vector-borne illnesses of concern are eastern equine encephalitis virus (EEE) and West Nile virus (WNV). Both are major causes of equine encephalitis, have similar clinical signs, and share similar modes of transmission. The Michigan State University Veterinary Diagnostic Laboratory (MSU VDL) offers several diagnostic tests to assist practitioners in identifying the cause of equine encephalitis. While there currently is no cure for EEE or WNV infections, both diseases are preventable in equine populations through routine vaccination.
Arboviruses, like EEE and WNV, are the most common cause of equine encephalitis. Horses are an important indicator species because increases in EEE and WNV infections in horses often correlate to outbreaks in humans. EEE and WNV outbreaks tend to occur in mid- to late summer and continue through fall. While horses account for over 95% of all mammalian, non-human WNV infections, most horses infected with WNV will not develop clinical disease. In contrast, outbreaks of EEE in the equine population are often accompanied by high fatality rates (80-90%). Other causes of encephalitis include viruses such as the alphavirus that causes western equine encephalitis (WEE), the rabies virus or equine herpes viruses; the protozoans Sarcocystis neurona and Neospora hughesi; and bacterial and nematode infections. A link to detailed data on equine encephalitis can be found at the USDA horse disease information web site. Of note, although the number of cases of western equine encephalitis (WEE) has dropped significantly over the last several years, this disease still should be considered as a differential diagnosis for equine encephalitis, especially in the western United States.
Prevalence of EEE and WNV
Since the USDA began collecting data in 1999, over 25,000 cases of WNV infection have been reported in horses, accounting for 96.9% of all non-human mammal WNV infections. In recent years, we have seen a steady decrease in the number equine WNV. There have been occasional outbreaks in both 2012 (690 equine cases) and 2016 (380 equine cases). In 2017, the USDA reported 317 equine cases. WNV is spread throughout North, Central, and South America and is heading north.
EEE infection in horses remains at a steady enzootic level with slight increases in 2005 and 2010. Since data collection began in 2004, there have been 2,362 equine cases reported in the United States. Last year, 86 equine cases were reported in 13 states. While both WNV and EEE can be found throughout the United States, EEE tends to be more prevalent in the eastern U.S.
Illustrating the ability of arboviruses to push north and affect susceptible species, of Michigan’s seven equine EEE cases in 2017, three were in the central Upper Peninsula in Marquette and Menominee counties—the first-ever reported mosquitotransmitted equine diseases in the Upper Peninsula, while the remaining four were in the central Lower Peninsula in Clair, Roscommon and Wexford counties. The number of Michigan’s equine WNV cases for 2017 is much higher than in previous years. APHIS only reported one case of equine WNV in Michigan in 2016 and three cases of equine WNV in Michigan in 2015, while there were 15 cases in 2017.
According to Dr. Tom Cooley with the Michigan Department of Natural Resources’ Wildlife Disease Laboratory, “Arboviruses have been in Michigan since 2001 and they are here to stay.”
Cooley’s department monitors WNV in wild mammal and bird populations—wild birds are a reservoir for both diseases. According to the data collected by Dr. Cooley, 224 WNV infected birds were identified in 64 counties across Michigan in 2017. This was the first time that WNV has been detected in birds in every county in the Upper Peninsula. In addition to birds, his group identified WNV positive white-tailed deer, Eastern fox squirrels, Eastern grey squirrels, elk, big brown bats and moose between 2002-2017.
Transmission and Risk of Infection
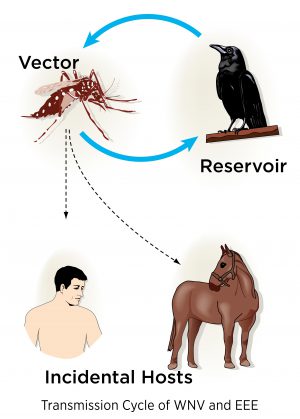
Both EEE and WNV must replicate in arthropod vectors, such as mosquitoes, hence the name arboviruses. Female mosquitoes acquire the virus while feeding on infected birds. The virus then replicates within the mosquito and is transmitted back into the bird population or into a variety of incidental hosts when the infected mosquito feeds. The risk of exposure varies from year to year and corresponds to changes in the mosquito populations.
More than 23 different species of mosquitoes can harbor and transmit the EEE virus with the Aedes and Culiseta species being the most common. The Culex mosquito is the most common mosquito to transmit WNV. Horses, like humans and other mammals, are an incidental or “dead-end” host meaning that they do not have a high enough viral load to pass the disease easily to other mammals or back into the mosquito population. Birds, on the other hand, are reservoirs for the disease.
Certain bird populations are much more susceptible to WNV infection than others. For example, the American crow, jays and yellow-billed magpies suffer a high mortality rate due to WNV infection. These birds experience high titer viremia accompanied by widespread tissue necrosis, hemorrhage, and inflammation of internal organs. Birds that do not experience high viremia during WNV infection such as robins, finches, and sparrows, maintain the virus in the wild.
Equine populations more susceptible to WNV infection include unvaccinated horses, foals, and horses over the age of 15. Other mammals are susceptible to WNV infection, but they tend to account for less than 5% of mammalian WNV infections. Some examples include household pets such as cats and dogs; livestock such as cattle, sheep and alpacas; and wildlife including bats, skunks, raccoons, and even alligators.
EEE virus primary reservoirs are birds, rodents, and snakes. EEE is most virulent in humans and horses; however non-native birds such as pheasants and domestic turkeys can also experience high mortality rates from infection. Smaller outbreaks have been observed in ostriches, emus, rock doves, house sparrows, and penguins. Most of these birds experience viscerotropic disease leading to necrosis of the parenchymal organs. Pheasants are the exception and tend to develop encephalitis. In other mammals, EEE infections have been found in sheep, deer, goats, cattle, camelids, dogs, and pigs.
Clinical Signs and Mortality
Arbovirus infections share similar symptoms during the initial stages of the infection; however the clinical signs and disease progression vary between EEE and WNV. In the beginning of an arbovirus infection, horses become quiet and depressed, and often have a fever.
For EEE, neurological symptoms appear approximately 9-11 days after infection. Clinical signs include altered mentation, impaired vision, aimless wandering, head pressing, circling, inability to swallow, irregular gait, muscle weakness, paralysis, and seizures. Once these neurological symptoms set in, most horses will become recumbent within 12-18 hours. EEE progresses rapidly and most deaths occur within two to three days after the onset of neurological symptoms. The mortality rate for EEE infections in horses is 80-90%.
The initial clinical signs of WNV typically include a low-grade fever, lack of appetite, and depression. Clinical signs of WNV vary greatly as the disease progresses. Approximately 90% of affected horses will have symptoms of infection in the spinal cord such as proprioceptive defects, ataxia and/or weakness; 40-60% will exhibit behavioral changes; and 60-90% will exhibit muscle tremors in the face and neck. WNV infections include the same neurological signs listed for EEE plus colic, lameness, anorexia, and fever. The mortality rate for WNV in horses is 35-45%. Survivors of either infection often have lasting neurological impairments.
Diagnostic Options
The best way to identify the cause of neurological disease antemortem is through serological testing. With EEE, IgM antibodies appear early on during infection. Due to the rapid progression of the disease, the animal typically dies before results can be reported. Only a few labs offer an IgM capture ELISA for EEE. The MSU VDL forwards serologic, antemortem samples to the National Veterinary Services Laboratory for EEE testing.
The MSU VDL offers a WNV IgM capture ELISA to detect early exposure to WNV. This test is specific for equine IgM. Serum is the best sample to submit for this test. The WNV capture ELISA is not recommended for horses that have been previously vaccinated for WNV because the horse’s immune system makes antibodies to the vaccine which results in a false positive result on the ELISA. If a horse has been vaccinated for WNV and is showing neurological symptoms, a WNV virus serum neutralization test is recommended. This test is also available at the MSU VDL.
Post mortem identification of EEE or WNV can be done by reverse transcription polymerase chain reaction (PCR). The advantage to PCR is that it has the ability to detect viral genetic material in fluids or tissues. During this process, a specific portion of the RNA viruses are converted to DNA, then millions of copies of that specific portion are copied. When sending in samples for either of these tests it is best to send the entire brain when possible. This lets our expert pathologists find lesions where the virus replicated which helps the laboratory correctly identify the disease. If it is impossible to submit the entire brain, the next best sample is a portion of the brain that includes sections of cerebrum and cerebellum from both halves of the brain and as much of the brain stem as possible. Both fresh and frozen tissues are acceptable sample types for these PCRs. Submitting fresh tissue is preferred if histopathology evaluation is requested to rule out other causes of neurologic disease. Cerebral spinal fluid is also another acceptable specimen type for these PCRs.
Treatment and Prevention
Beyond supportive therapy, treatment options for arbovirus infections are limited. Some studies suggest that IFN-α can be used as a treatment aid to boost the horse’s immune system during a WNV infection. Because treatment options are severely limited, prevention is the best strategy, and this can be accomplished through vaccination. The American Association of Equine Practitioners (AAEP) recommends the following:
- Annual EEE and WNV vaccinations should be completed in the spring, prior to the summer months when likelihood of infection is at its highest.
- Animals with an unknown EEE vaccination history should receive a primary series of 2 doses with 4-6 weeks between the first and second dose. WNV vaccination follows similar guidelines.
For specific details on recommended vaccination protocols please visit the AAEP’s website.
In addition to vaccination, controlling the mosquito population will also help fight disease. Besides removing standing water (e.g. cleaning gutters, covering or turning over wheelbarrows or feeders not in use) and using pesticides, the MSU Extension program suggests some natural strategies veterinarians can recommend to owners to help control insect populations:
- Remove manure from stalls and pastures;
- Keep water supplies bug free by adding a few fish to eat mosquito larva;
- Provide a fly sheet and/or mask; and
- Introduce natural predators such as bats.
Increased Risk for Eastern Equine Encephalitis for 2019
Summer 2019 Update from the Michigan Department of Agriculture and Rural Development (MDARD):
Excessive rainfall this spring and summer have heightened concerns about eastern equine encephalitis (EEE). Additionally, disease trends have shown that in Michigan equine outbreaks of EEE can occur every 9-10 years. The last outbreak in 2010 involved 56 confirmed cases.
It’s not too late to vaccinate. MDARD urges horse owners to work with their veterinarian to safeguard their horses from EEE and West Nile virus (WNV).
Both diseases are commonly seen from summer to late fall and are spread by mosquitoes. Mosquitoes carrying WNV have already been detected in Michigan and while mosquitoes are not typically tested for EEE, cases are seen each year, especially in Southwestern Michigan and the Upper Peninsula. Both diseases cause neurologic issues, and EEE is particularly concerning, as horses with the disease have a low chance of survival.
References:
American Association of Equine Practitioners. External Parasite and Vector Control Guidelines: Mosquitoes, https://aaep.org/sites/default...
American Association of Equine Practitioners. Vaccine Guidelines for WNV and EEE, https://aaep.org/guidelines/va...
Fenner’s Veterinary Virology, 5th edition p. 511-545.
Go, Y. Y., Balasuria, U.B.R., Lee, C. (2014) Clin Exp Vaccine Res. 3: 58-77.
Krow-Lucal E, Lindsey NP, Lehman J, Fisher M, Staples JE. West Nile Virus and Other Nationally Notifiable Abroviral Diseases-United States, 2015. MMWR Morb Mortal Wkly Rep 2017; 66: 51-55.
Long, M.T. Overview of Equine Arboviral Encephalomyelitis, Merck Manual
Long, M.T. West Nile Virus and Equine Encephalitis Viruses, Vet Clin Equine (2014) 30: 523-542
Michigan State University Extension. 5 Tips for protecting your horse from insects, http://msue.anr.msu.edu/news/t... .
United States Department of Agriculture Animal and Plant Health Inspection Service, Veterinary Services, West Nile Virus - States with Equine Cases (link removed)
United States Department of Agriculture Animal and Plant Health Inspection Service, Veterinary Services, Eastern Equine Encephalitis - States with Equine Cases, https://www.aphis.usda.gov/liv...
Van der Meulen, K.M., Pensaert, M.B. and Nauwynk, H.J. West Nile Virus in the Vertebrate World, Archives of Virology (2005) 150: 637-657.
