When we’re sick, we rely on our immune system to fight off what’s making us sick. Antigens are tiny foreign molecules—part of the “bad guys”—that signal to our body that these cells are a threat and need to be destroyed. Then, our bodies produce antibodies that tag antigens for elimination. At the same time, our immune systems can send out T cells to destroy the threatening cells.
This is usually an effective process, but cancer is more evasive than most other diseases. According to the National Cancer Institute, cancer cells may limit their expression of antigens. This means the body has a hard time tagging these cells for destruction because cancer cells can hide themselves. Cancer cells also may produce proteins that deactivate the immune system or attract other cells that suppress immune responses, all while promoting tumor cell development and vitality.
But the cancer cells’ antigens also may be their own undoing. That’s according to Dr. Xuefei Huang, MSU Foundation professor and associate chair in Research for the Departments of Chemistry and Biomedical Engineering with MSU IQ. He is the primary investigator on a new research grant awarded by the National Cancer Institute. Huang is working with the MSU College of Veterinary Medicine’s Drs. Vilma Yuzbasiyan-Gurkan, cancer researcher and associate dean of Research and Graduate Studies, and Paulo Vilar Saavedra, head of the Oncology Service for the MSU Veterinary Medical Center.
“Cancer is the second leading cause of death in the world,” says Vilar Saavedra. “Developing an effective anti-cancer vaccine is an attractive way to treat and prevent cancer and its recurrence.”
In their new grant titled “Engineering bacteriophage Qβ conjugates with tumor associated carbohydrate antigens as multi-component anti-cancer vaccines,” which awards $2,409,326 from January 1, 2018–December 31, 2022, Huang, Yuzbasiyan-Gurkan, and Vilar Saavedra will focus on engineering Qβ to generate powerful anti-cancer immune responses.
How the vaccine will work
Cancer is challenging in many ways. It’s difficult for conventional treatments like chemotherapy to wipe out all cancer cells. When there are cancer cells left over after treatment, the disease can reoccur or migrate to new places in the body and cause metastatic disease.
“To truly wipe out cancer cells, we must harness the immune system,” says Yuzbasiyan-Gurkan.
Immune cells have the ability to go and find cancer cells by recognizing their antigens, which they normally do, and then destroy them. But successful cancers suppress the immune system by secreting proteins that cloud signals that would normally trigger an immune response. To help combat this, the researchers are focusing on a special type of antigen that is present in various types of cancer cells.
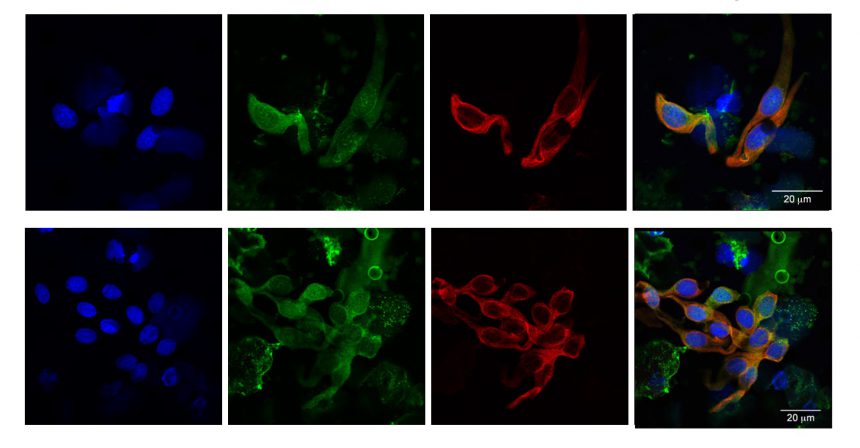
“In this research, we’re targeting tumor-associated carbohydrate antigens (TACAs) to use in anti-cancer vaccines,” says Huang. “Many TACAs are highly expressed on multiple types of cancer cells with no or negligible expression on normal cells. This helps them stand out to the body’s immune system.”
The problem with TACAs is that their natural ability to initiate an immune response in a host isn’t enough; the immune system is still unable to identify the cancer antigen on its own. The researchers are addressing this challenge by attaching a bacteriophage— Qβ—to a TACA called Tn. The immune system already recognizes Qβ as a threat. When Qβ is attached to Tn, Qβ is able to flag down the immune system and present Tn as a threat. Once that happens, the immune system kicks in and begins creating the antibodies necessary to tag the cancer cells with the Tn antigen for destruction.
“We’ve found that bacteriophage Qβ is a superior platform for the Tn antigen,” says Yuzbasiyan-Gurkan. “The Qβ-Tn immunogen, which can be bound by the host’s immune system, has a high rate of success in generating anti-Tn antibodies. This allows the host to be immunized.”
The TACAs will promote long-lasting antibodies and T cells dedicated to fighting cancer in a newly invigorated immune response. This also will help the body’s immune cells fight against recurring cancers.
“There’s no better disease fighter than the immune system; drugs and other therapies can leave cancer cells behind, and then there’s nothing left to fight recurrence,” says Huang. “Our vaccine would reduce tumor growth and protect the host against tumor development and redevelopment. If we can further understand the connections between the structural features of Qβ-TACA conjugates and anti-tumor immunity, we can make a sustained impact on cancer vaccine design.”
This research also emphasizes the importance of veterinary medicine in cancer research.
“Spontaneous cancers in dogs and cats provide a true test for this cancer vaccine approach,” says Yuzbasiyan-Gurkan. “This is just one example of the many ways that veterinary and human medical research can benefit each other.”
