How a Blood-Clotting Protein Signals Regeneration in Liver Resection Patients
The Luyendyk Laboratory
The Luyendyk Laboratory at the MSU College of Veterinary Medicine studies the mechanisms connecting blood coagulation and liver disease.
“In an emergency, first responders need to be in the right place at the right time.”
One might not expect a scientist to describe their research this way, but Dr. James Luyendyk of Michigan State University draws this comparison for recovery after liver resection.
“After liver resection (also called partial hepatectomy [PH]), a surgery where a diseased piece of the liver is removed, we know that cells in the blood called platelets accumulate in the remaining healthy liver and release factors to help it regenerate,” says Luyendyk.
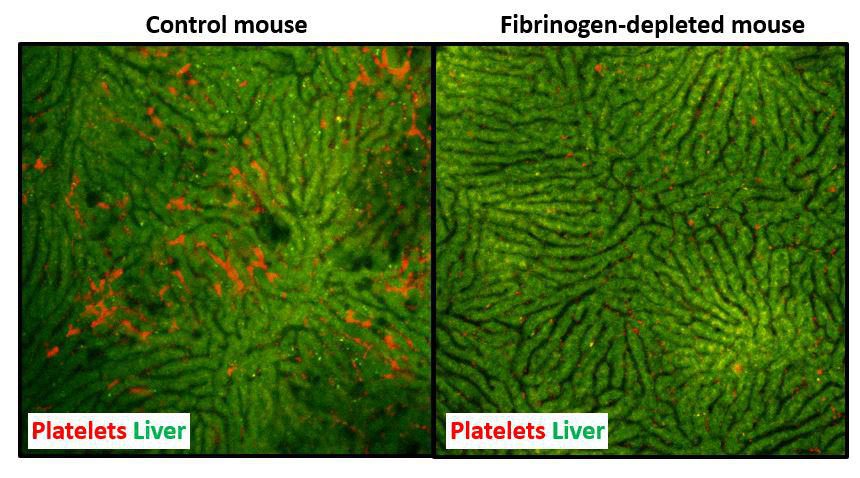
The liver is unique among other organs, in that it can regenerate to maintain normal function, even after removal of as much as 70 percent of its original size. Successful regeneration of the remaining liver is essential to maintain normal health after PH. Platelets play a vital and established role in liver regeneration after PH; when platelets are inhibited, liver regeneration is delayed.
“Platelets are effectively first-responders, delivering aid to the remaining liver, signaling for it to regenerate,” Luyendyk says. “Much like first-responders in an emergency, platelets require local direction on where and when to release their pro-regenerative cargo.”
While the exact mechanisms linking platelets to liver regeneration are a topic of continued experimentation, a discovery made by Luyendyk’s laboratory suggests that a specific component of the blood clotting pathway promotes the accumulation of platelets within the liver.
“We discovered that platelets seem to receive this instruction from a blood clotting protein called fibrinogen, which is deposited in the liver in the earliest moments after PH,” Luyendyk says.
International collaboration
For this research, Luyendyk and Groeneveld worked with Dr. Patrick Starlinger, associate professor and consultant for surgery for the Medical University of Vienna’s Department of Surgery in Vienna, Austria, and Dr. Ton Lisman, professor in experimental surgery for the University Medical Center Groningen’s Surgical Research Laboratory and Section of Hepatobiliary Surgery and Liver transplantation in Groningen, Netherlands.
This discovery is reported by Luyendyk, a professor for the MSU College of Veterinary Medicine’s Department of Pathobiology and Diagnostic Investigation, and Dr. Dafna Groeneveld, a postdoctoral research associate in Luyendyk’s Laboratory, with their key collaborators, Dr. Ton Lisman of the University Medical Center Groningen and Dr. Patrick Starlinger of the Medical University of Vienna, in their latest paper entitled “Intrahepatic fibrin(ogen) deposition drives liver regeneration after partial hepatectomy in mice and humans.
According to their paper, now published in Blood, the official journal of the American Society of Hematology, fibrin deposits can be detected in the liver as soon as 30 minutes post-PH. The authors observed that when fibrin deposits were prevented in mice undergoing PH, platelet accumulation in the liver was reduced as well; as a result, liver regeneration was delayed. This observation revealed that the presence of fibrin deposits directly impacts liver regeneration post-PH.
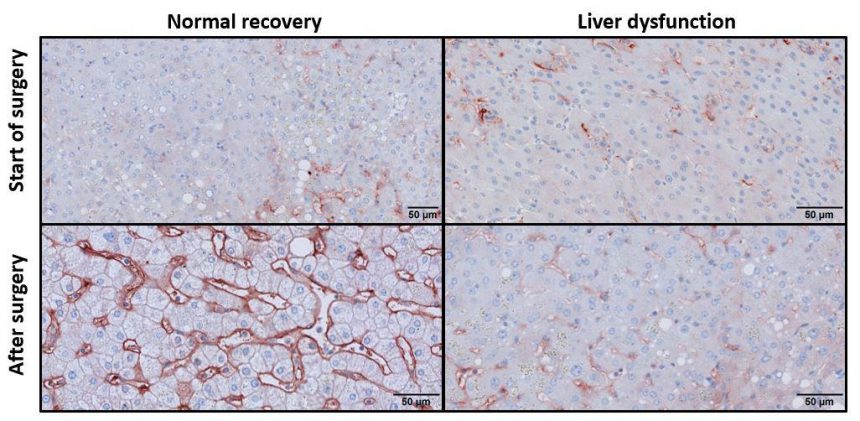
Their conclusion, based on experiments in mice, aligned flawlessly with observations in human patients, says Luyendyk. Using liver samples from patients that underwent liver resection at the Medical University of Vienna in Austria, Starlinger’s research team discovered that patients with increased fibrin deposits post-PH showed healthy liver regeneration, whereas a lack of fibrin deposits post-surgery marked patients who later experienced liver dysfunction.
“We don’t perceive this as anecdotal,” says Luyendyk. “It’s a core translational observation.”
Groeneveld agrees. “This demonstrates real potential as a predictive marker,” she says. “If we can measure fibrin levels in PH patients, we may be able to determine in advance whether their liver will regenerate successfully or if they will experience liver dysfunction.”
The potential for diagnostics and therapeutics
Dr. Dafna Groeneveld
From 2008–2013, Groeneveld completed her PhD studies on hemostasis-related topics involving von Willebrand factor (VWF) at the Department of Thrombosis and Hemostasis at the University Medical Center, Leiden in the Netherlands. Groeneveld then joined the Surgical Research Laboratory at the University Medical Center, Groningen in the Netherlands in 2013 as a postdoctoral researcher, where her work focused on clinical and fundamental aspects regarding the interface between hematology, hepatology, and surgery.
During postdoctoral studies in Dr. Ton Lisman’s laboratory, she spent three weeks at the MSU College of Veterinary Medicine, where she began the research that led to the eventual publication of the paper “Intrahepatic fibrin(ogen) deposition drives liver regeneration after partial hepatectomy in mice and humans.” Her experience was made possible by the College’s Visiting Scholar Funds.
Since October 2017, Groeneveld has worked as a postdoctoral research associate in Dr. James Luyendyk’s Laboratory. Her current research focuses on the complex relationship between coagulation and liver disease, and is funded by a grant from the European Hematology Association.
In some patients, insufficient or uninitiated liver regeneration is a major contributing factor to developing liver failure post-liver resection. Liver failure remains one of the most serious complications following PH, and is a significant source of morbidity and mortality. These patients then likely face one of two outcomes: they must receive a liver transplant, or the liver insufficiency could be fatal.
Luyendyk and Groeneveld’s new discovery on the role of fibrin in liver regeneration could change that. It is possible that new approaches centered on fibrin could be developed to predict which patients will undergo successful liver regeneration after the surgery.
“Measuring intraoperative changes in fibrin could predict future liver dysfunction, whereas right now, testing and monitoring of coagulation factors in patients is typically done days after surgery,” says Groeneveld. “Our results suggest the appearance of fibrin—an easily measurable protein—in the liver predicts right away whether or not the stage is set for successful liver regeneration.”
Moreover, the research team sees real potential in correcting defects in liver fibrin levels that could predispose patients to liver dysfunction. “There are approved therapeutic strategies based on increasing plasma fibrinogen levels,” says Luyendyk. “They just haven’t been tried in PH patients. We believe our published and ongoing studies will ultimately provide the proof-of-concept needed to translate these concepts to the clinic.”
For more information on this research, contact Dr. Jim Luyendyk.
Funding provided by visiting scholar funds
Dr. Lisman’s and Dr. Groeneveld’s visits to Michigan State University were funded by the College of Veterinary Medicine’s Visiting Scholar Program. These funds support the College’s international colleagues while they visit MSU to learn new techniques, share ideas and discoveries, and collaborate in ongoing research initiatives at the College.
