At Michigan State University, researchers in the Luyendyk Lab are uncovering a hidden layer of biology that could reshape how we understand — and treat — trauma, wound healing, and chronic disease. Their latest work reveals a surprising twist in the story of how blood clots form and function, and could lead to new therapies for conditions ranging from traumatic tissue injury to pathologic blood clots (known as thrombosis).

A Hidden Architect of Blood Clots
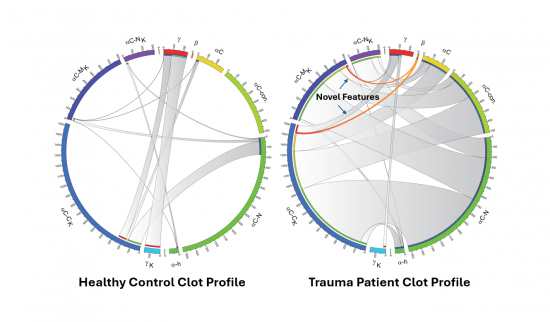
Blood clotting is a vital defense mechanism within the body. When blood vessels are injured, a protein known as fibrin forms a mesh that stops bleeding and begins the healing process. The structure of this mesh, the blood clot, is stabilized by an enzyme called Factor XIII.
“Factor XIII crosslinks fibrin together like bolting pieces of lumber together,” says Nana Kwame Kwabi Boateng, a PhD student in the Luyendyk Lab.
Luyendyk’s team discovered that another enzyme, tissue transglutaminase (TG2), plays a previously unknown role in reshaping fibrin’s structure during trauma. “We found that TG2 bolts fibrin together differently than Factor XIII does,” says Boateng. “It’s like a completely different blueprint in trauma clots.”
This breakthrough centers on the β-chain of fibrin, a piece of the fibrin protein never shown to be crosslinked by these enzymes. As published recently in the leading hematology journal, Blood, the team found that TG2 crosslinks this chain in trauma patients, creating clot architectures never before seen in healthy individuals.
“If you have blood, this discovery could one day save your life."
Why It Matters
These findings are more than academic. Traumatic injury is a major cause of morbidity and hospitalization in both humans and companion animals. There are still many unknowns on how the blood clotting system behaves when the body undergoes the intense challenge of healing, and every discovery is a step toward better patient care.
The results suggest a unique clotting mechanism that could impact the severity of bleeding, wound healing and long-term damage after traumatic injury.
The implications for patients are vast:
- New diagnostics: TG2-driven crosslinking patterns (i.e., the fibrin construction blueprint) could serve as biomarkers for trauma severity.
- Therapeutic innovation: Targeting TG2-mediated crosslinking (i.e., changing where TG2 can drive the bolts) may help prevent bleeding and limit tissue damage.
- Better recovery: Insights from this research could improve wound healing and post-surgical outcomes in both humans and animals.
One Health: A Collaborative Effort
This research is a collaboration between experts in trauma surgery, toxicology, hematology, and biochemistry — encompassing institutions like the University of Colorado, Denver Health Medical Center, and Rutgers University. MSU’s unique integration of veterinary medicine, toxicology, coagulation biology, and liver injury research positions it as a leader in this field.
The study also exemplifies the One Health approach, bridging human and veterinary medicine. The mechanisms uncovered apply across species, offering potential improvements in surgical care and recovery for both people and animals.
According to Boateng, “If you have blood, this discovery could one day save your life.”
