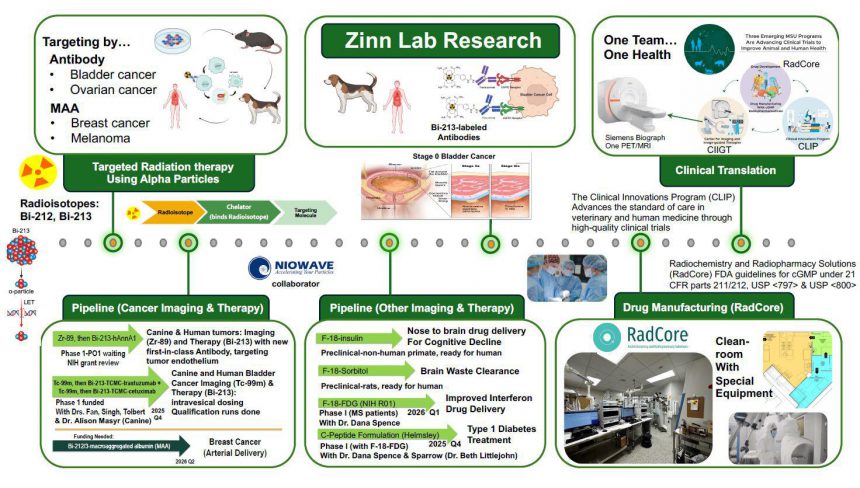
The Zinn Lab conducts translational research that bridges companion animal and human health, with a particular emphasis on cancer imaging and therapy.
A major focus of the laboratory is the development of radiopharmaceuticals for both diagnostic and therapeutic applications. Among these, targeted alpha particle therapy (TAT) has emerged as a promising strategy for treating advanced cancers with limited therapeutic options. Bi-213–labeled trastuzumab and cetuximab have demonstrated strong efficacy in eliminating both canine and human bladder cancer cells, and TAT is also being explored as a viable treatment for ovarian cancer and solid tumors such as melanoma and breast cancer.
To support these efforts, Dr. Kurt Zinn and his team have established a cGMP-compliant radiopharmacy for the production of drugs and radiopharmaceuticals used in clinical studies. This facility is a core component of RadCore (Radiochemistry and Radiopharmacy Solutions), a recently funded shared resource. Dr. Zinn serves as Director of RadCore, as well as Director of the Clinical Innovations Program, a donor-supported initiative aimed at improving standards of care in companion animals and humans through clinical trials.
The Zinn Lab collaborates closely with the MSU Drug Development group (Dr. Andrea Gonzales and Dr. Edmund Ellsworth) to radiolabel novel therapeutics for imaging studies, and with Dr. Dana Spence to evaluate a C-peptide formulation for the treatment of Type I diabetes. In addition, Dr. Zinn is helping to establish the Center for Imaging and Image-Guided Therapies (CIIGT), which will house a research PET/MRI system for imaging studies in companion animals, large animals (including pigs and sheep), and humans. This effort is supported by substantial institutional investment from the Department of Radiology (Dr. Mark DeLano and Dr. Erik Shapiro) and a funded NIH C06 grant (Dr. Anna Moore).