Adam J. Moeser, DVM, PhD, associate professor and Matilda R. Wilson Endowed Chair, is part of a group of researchers who are the first to uncover reasons why a specific type of immune cell acts very differently in females compared to males while under stress, resulting in women being more susceptible to certain diseases.
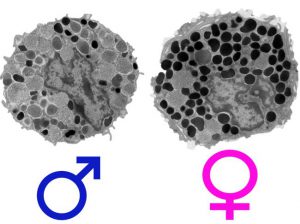
In the study, females were found to be more vulnerable to certain stress-related and allergic diseases than males because of differences found in a type of white blood cell that’s part of the immune system.
The National Institutes of Health funded study found that females were more vulnerable to certain stress-related and allergic diseases than males because of distinct differences found in mast cells, a type of white blood cell that’s part of the immune system.
“Over 8,000 differentially expressed genes were found in female mast cells compared to male mast cells,” Moeser said. “While male and female mast cells have the same sets of genes on their chromosomes, with the exception of the XY sex chromosomes, the way the genes act vary immensely between the sexes.”
The study is co-authored by Emily Mackey, a doctoral student in veterinary medicine, and is published in the journal Biology of Sex Differences.
Mast cells are an important immune cell because they play a key role in stress-related health issues that are typically more common in women such as allergic disorders, autoimmune diseases, migraines and irritable bowel syndrome, or IBS.

IBS, for example, is a disorder in the intestine that creates significant abdominal pain and affects almost a quarter of the U.S. population. Women are up to four times more likely to have it than men.
A further in-depth analysis of the genes within the RNA genome – a primary building block in all forms of life – revealed an increase in activity that’s linked to the production and storage of inflammatory substances. These substances can create a more aggressive response in the body and result in disease.
“This could explain why women, or men, are more or less vulnerable to certain types of diseases,” Moeser said.
With this new understanding of how different genes act, Moeser said scientists could eventually start developing new sex-specific treatments that target these immune cells and stop the onset of disease.
He added though that an important next step in his research is figuring out when in the development stage these immune cells start to act differently.
“Pinpointing when this variance happens will let us know if it occurs during adulthood or in individuals at an early age,” Moeser said. “Many mast cell diseases exhibit a sex bias in children and if we can identify the timing and the mechanism of what’s influencing the change, we’ll have an even better understanding of how these immune cells cause disease and know when to intervene with potentially new therapies.”
This article reprinted from MSU Today: http://msutoday.msu.edu/news/2...
Dr Moeser's Research Lab Website.
Original publication:
Mackey E, Saravanan A, Pohl CS, Costa SD, Yihang L, Moeser AJ. Sexual dimorphism in the mast cell transcriptome and the pathophysiological responses to immunological and psychological stress. Biol Sex Differ. 2016 7(60).
