By Brea Sandness, surgery resident for the MSU Veterinary Medicine Center, and Anna-Marie Struble, DVM Class of 2020 student at the MSU College of Veterinary Medicine
Departments involved: clinicians, licensed veterinary technicians, and veterinary medicine and veterinary technology students in MSU Emergency and Critical Care Medicine Service, MSU Comparative Ophthalmology Service, and MSU Soft Tissue Surgery Service.
History and Presentation
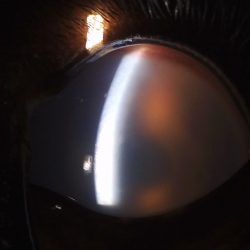
Stella, a 37-kilogram (81.5 lbs.), 18-month-old female intact Rottweiler presented to the Michigan State University Veterinary Medical Center’s Emergency and Critical Care Medicine Service (ECCM) after being found by the Lansing Fire Department in a house fire on Wednesday, February 13, 2019. On presentation, Stella was quiet, alert, and responsive; she was tachypneic and displayed increased effort. She also had increased lung sounds along both hemithorax and facial swelling around both eyes. Her body temperature was normothermic (99.9⁰ F). She had full-thickness dermal burn wounds on the right ventral thorax and inguinal, perivulvar, periocular, and nasal regions; coverage was estimated at 10 percent of her body surface area. Full oral examination, abdominal palpation, and rectal examination were unable to be performed due to respiratory compromise. No fluorescein stain uptake was noted bilaterally; however, there was hyphema-noted OU. Patient smelled of smoke and burnt hair, and was covered in soot diffusely. Remainder of physical examination was within normal limits.
stella in the news
Diagnosis
Patient was administered Methadone IV and thoracic radiographs were performed, which showed increased bronchointerstitial pattern in caudodorsal lung fields bilaterally with lobar signs visible on lateral projections. Based on board-certified radiologist interpretation, radiographs indicated severe smoke inhalation, noncardiogenic pulmonary edema, tracheal changes consistent with tracheal wall edema/hemorrhage, and tracheitis from thermal injury.
A venous blood gas indicated a lactic acid/metabolic acidosis (pH=7.20; calculated HCO3=13.9 mmol/L, reference interval 16.0-25.0 mmol/L; lactate=10.2mmol/L, reference interval 0.2-3.3 mmol/L), mild hypercalcemia (5.4 mg/dL, reference interval 3.4-5.3 mg/dL), and moderate hyperglycemia (269 mg/dL, reference interval 66-117 mg/dL). She was mildly hemoconcentrated with a packed cell volume of 57 percent and a total solids of 7.8 g/dL.
Based upon the history and clinical presentation, Stella was diagnosed with smoke inhalation, full- and partial-thickness dermal burns (~10% BSA), and bilateral corneal edema.

Treatment
Stella was immediately placed within an oxygen-saturated kennel and treated with amoxicillin/sulbactam [30 mg/kg IV Q8 hours (Unasyn; Pfizer, New York City, New York, USA)]; topical with silver sulfadiazine cream (Q4 hours); eye lubricant (OU Q4 hours); neomycin, polymyxin B, and bacitracin ophthalmic ointment (OU Q6 hours); ofloxacin ophthalmic drops (1 drop OU Q12 hours); fentanyl (2-5mcg/kg/hr IV constant rate infusion); maropitant [1mg/kg IV Q24 hours (Cerenia; Zoetis, Parsippany, New Jersey, USA)]; enrofloxacin [350 mg IV Q24 hours (Baytril; Bayer, Shawnee Mission, Kansas, USA)]; and trazodone (100 mg PO Q12 hours). She was provided supplemental oxygen with oxygen values between 30 and 50 percent, and was provided coupage and terbutaline treatments (0.01mg/kg intramuscularly Q12 hours). Procedures were occasionally completed using acepromazine and methadone due to respiratory compromise and dyspnea. On Monday, February 25, 2019, Stella was able to be removed from the oxygen cage and bilateral nasal oxygen lines were placed under mild sedation with acepromazine and methadone. At that time, wound assessment of the right ventral thoracic/axillary was performed by the MSU Soft Tissue Surgery Service (STS) and minimal debridement was performed by the MSU ECCM as tolerated at that time. Throughout her hospitalization, Stella lost approximately 6 kg of bodyweight (~13 lbs.).

On Wednesday, February 27, 2019, Stella was placed under mild sedation with acepromazine and methadone and continued on supplemental oxygen. The right ventral thorax burn was minimally debrided and prepared for the Kerecis™ Omega3 BURN (Kerecis, Isafjordur, Iceland) graft. Kerecis™ Omega3 BURN was rehydrated in sterile saline for one minute, trimmed, and applied onto the wound as recommended by the company. The graft was stapled into place along the margins of the wound. The graft was covered with Adaptic (KCI, San Antonio, Texas, USA), polyurethane foam, and cotton 4x4s (or laparotomy sponges), and then secured with a tie-over bandage. This was how the application was recommended by the Kerecis™ company and kept consistent throughout the remainder of her graft applications and bandage care.
Stella remained in the Hospital on oxygen supplementation following her first graft placement. On Friday, March 1, 2019, Stella was administered acepromazine and methadone once again for wound and graft assessment. Her tie-over bandage had fallen off earlier in the day, and upon examination, the graft was difficult to visualize, except for underneath the staples along the peripheral caudal. There was continued peri-wound erythema and swelling. Previously noted partial-thickness burn caudal to the larger full-thickness burn showed eschar that needed debridement. Increased granulation tissue was present in the cranial ventral aspect of the right axillary region. Superficial burns present caudally to the larger wound showed changes, as they appeared darker and harder. Due to the presence of eschar, it was elected to perform debridement under minimal sedation (Acepromazine and Methadone) to remove the eschar, and consequently, the remaining visible graft. Due to concern for continued declaration of the wound, it was elected to not place an additional graft at that time. No new bandage was placed at that time.
Stella was stable for 24 hours without oxygen supplementation. She was discharged from the MSU ECCM on Saturday, March 2, 2019 with Cerenia [1.9 mg/kg PO Q12 (Zoetis, Parsippany, New Jersey, USA)], Clavamox [12 mg/kg PO Q12 (Zoetis, Parsippany, New Jersey, USA)], Tramadol HCl [ 3.23 mg/kg PO Q8-Q12 (Ultram, Raritan, NJ)], Enrofloxacin [10.96 mg/kg PO Q24 (Baytril; Bayer, Shawnee Mission, Kansas, USA)], ofloxacin ophthalmic drops (1 drop OU Q6) and OptixCare eye lubricant [1/4-inch strip OU Q4 (Aventix company, Canada). As there was no bandage in place, she was discharged with instructions for wound care at home. Owners were instructed to lavage the wound bed gently at home with shower head for 5–10 minutes daily, and to dry periwound with clean towel and wound bed with cotton. Following drying, owners were to spray Vetericyn Plus (Innovacyn, Inc. Rialto, CA) on the wound bed once daily and a clean t-shirt was to be placed on top.
Following discharge, Stella returned on Monday, March 4, 2019 on an outpatient basis for an assessment of her wound and placement of the second Kerecis™ Omega3 BURN graft. The remainder of the larger wound appeared to declare itself. There was an island of hard, colder necrotic tissue caudally in the wound bed. There continued to be healthy granulation tissue present cranially in the wound bed. There was no longer any periwound erythema or swelling. Following minimal debridement of the necrotic tissue, the second Kerecis™ Omega3 BURN graft was placed in the same fashion as described earlier and the same combination of sedation was used as previously.
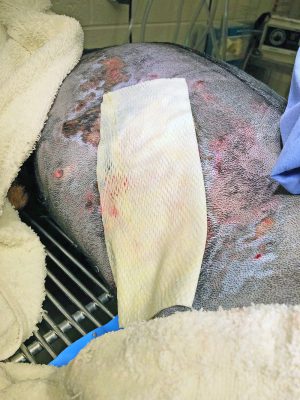
She was discharged and returned for an outpatient bandage change on Thursday, March 7, 2019. No sedation was used for the bandage change. There was a moderate amount of exudate saturating the previously placed laparotomy sponges. The bandage was removed and showed a thin layer of the graft present in the caudal aspect of the wound bed that was integrating well. The previously placed staples and sutures that held the graft were removed. No lavage was performed. There was no necrotic tissue present and a healthy, thin rim of epithelialization was on the ventral aspect of the wound bed. The wound bed was covered with Adaptic (KCI, San Antonio, Texas, USA), polyurethane foam, laparotomy sponges, and a tie-over bandage as described previously, and Stella was discharged.
She re-presented on Monday, March 11, 2019, as the bandage had continued exudate saturation of the laparotomy sponges. Upon removal, the second graft appeared almost completely incorporated and was difficult to grossly identify. There was continued granulation of the wound bed, and epithelialization around the edges of the wound. Periwound was cleaned with chlorohexidine solution and saline. Due to gentle lavage of the wound bed, 3 mg Hydromorphone was administered IV for mild sedation. Stella was discharged as an outpatient.
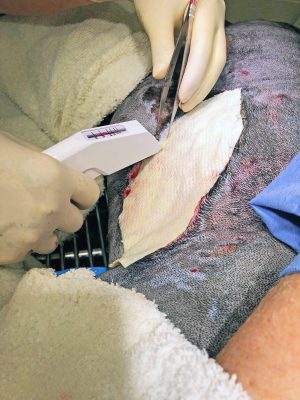
Stella returned on Thursday, March 14, 2019 for a final graft placement. At that time, the owner reported increased coughing at home and loss of appetite since the previous evening. Her temperature was mildly elevated at 102.9° F. There were increased respiratory sounds in both hemithorax along with audible crackles and wheezing. She had finished her last antibiotic (ClavamoxTM) approximately 3 days prior. Stella’s owner declined thoracic radiographs. Mild sedation was provided with 0.01 mg/kg Acepromazine and 0.2 mg/kg Methadone IV; flow by oxygen also was provided, and her previously placed bandage was removed. The wound continued to have healthy granulation tissue and epithelialization with contraction. The smaller wound bed caudal to the larger wound bed was almost completely epithelialized. The smaller superficial wounds surrounding the larger wound also had completely epithelialized. The wound was gently lavaged with sterile saline. The last Kerecis™ Omega3 BURN was applied in the same fashion as previously described. Stella was oxygenating well following bandage change.
The MSU Comparative Ophthalmology Service performed a recheck at that time and determined she still had significant corneal edema OD and prescribed Sodium Chloride 5 percent Ophthalmic Ointment (1 drop OD Q8). Stella was discharged with suspect recurrence of pneumonia due to her previously sustained thermal lung injuries, and two additional weeks of ClavamoxTM were prescribed.
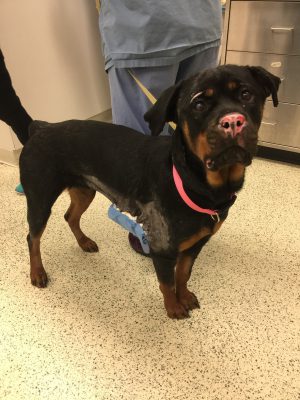
Stella rechecked on Monday, March 18, 2019, and had been doing well. No sedation was needed. There was a minimal amount of exudation present on the bandage. The previous bandage was removed along with the previously placed staples and sutures. There had been increased epithelialization and contraction. Both the length and the width had decreased since the initial graft placement on Wednesday, February 27, 2019. No lavage was performed. Telfa and hypafix were placed over the wound bed prior to her discharge, and were to be removed in 3–5 days, but fell off approximately 48 hours later. After they had fallen off, no bandage was replaced at that time and no topicals were recommended.
Stella returned on Wednesday, March 27, 2019 with decreased frequency of coughing/hacking at home and still on her previously prescribed ClavamoxTM. There was no bandage present and no topicals were being used. The wound bed was dry, except for a small 0.5 cm portion within the axilla. Wound bed had markedly contracted and epithelialized. There was growth of hair along the periphery. The Comparative Ophthalmology Service rechecked tear production, intraocular pressure, and dilated pressures; the corneal ulcers appeared to have healed bilaterally with continued corneal edema and mild to moderate keratoconus present bilaterally. Stella was discharged and continued on Sodium Chloride 5 percent Ophthalmic Ointment (1 drop OU Q8), OptixCare eye lubricant [1/4-inch strip OU Q4, and Clavamox (13 mg/kg PO Q12). She was instructed to continue use of the e-collar and recheck in one month to assess the wound. No bandages or topicals were needed.

Stella returned on Thursday, April 25, 2019. The wound had contracted significantly; the length measured at 1 cm and the width measured at 0.5 cm. Stella’s owner noted that she had increased episodes of waking up in the middle of the night coughing, irregular appetite, and she was quieter. She had been off of the previously prescribed antibiotic for approximately 1-month’s duration. On physical exam, she was normothermic (100.8° F) with mild-moderate harsh lung sounds in both hemithorax (right > left). She also had a wet-sounding cough. The remainder of her physical exam was unremarkable and unchanged. Owners approved thoracic radiographs. Three-view thoracic radiographs were performed with no sedation. Radiographs showed an alveolar pattern consistent with bacterial pneumonia along with a more generalized bronchial, unstructured interstitial, and band-like interstitial changes that are consistent with fibrosis and with the history of thermal injury and smoke inhalation. A thoracic fast scan was performed; it showed no evidence of pleural fluid. Stella was prescribed Clavamox (15.9 mg/kg PO Q12) for another 4 weeks and was recommended to have recheck radiographs performed in 3–4 weeks while still on the antibiotic and the continuation of coupage 3–5 times daily. Owners did not elect for more recheck radiographs; however, upon follow up, the owner reported that following completion of the antibiotics, Stella rarely coughed and only showed mild change in her activity tolerance when compared to the other dog in their home during playtime. She had had no changes in her appetite or attitude and no longer had a hacking cough.
Her wound healed approximately 3 weeks later (approximately mid-May 2019) according to the owner; however, Stella did not return for official documentation until Tuesday, June 11, 2019. At this recheck, the owner was extremely happy with her recovery and she was no longer on any ocular or oral medications. She also was not receiving any thoracic coupage. No rechecks with the MSU Soft Tissue Surgery Service were recommended at that time. If her respiratory signs were to return, then it was recommended that Stella be rechecked with the MSU Internal Medicine Service for potential bronchoscopy and bronchial alveolar lavage, if indicated.

Results
Wound length decreased by approximately 70 percent (20 cm to 6 cm) and width decreased by 90 percent (10 cm to 1 cm) 28 days from initial graft application and with 3 total applications of the graft. After 56 days from the initial application of the graft, wound length decreased by 95 percent (20 cm to 1 cm) and width decreased by 95 percent (10 cm to 0.5 cm). Grafts were placed anywhere from 5 and 10 days apart. Placement was determined by the amount of graft visible during recheck and by previously reported integration times that were studied in human medicine. Graft placements were kept standardized throughout the treatment period and were determined by the protocol set by the company. No debridement was needed after 1 week of the initial graft application. There was healthy granulation tissue in the wound bed by 1 week after initial application. Graft was noted to grossly integrate into the wound bed quickly. Throughout the use of the acellular fish skin omega-3 rich graft KerecisTM Omega3 BURN graft, there were no complications or adverse effects encountered.
Clinical Relevance and Discussion
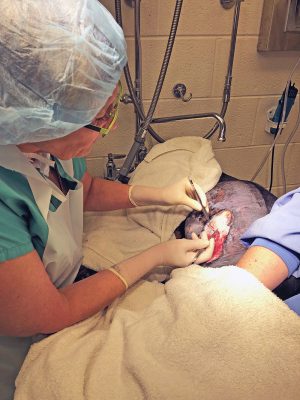
Thermal burn wounds remain relatively uncommon in veterinary practice. Causes include flame burns (accidental or deliberate), scalds (accidental or deliberate), automotive mufflers, hot stove surfaces, radiators, hot air dryers, electrical heating pads, hot water bottles, chemicals, hot packs, heat lamps, electrical cords, and improperly grounded electrocautery units9.
Burn injuries result from a series of pathophysiological events that are not encountered in other kinds of injuries15. Therefore, early and aggressive therapy, as well as long-term management, is imperative for the victim to survive. These treatments require treating not only the visible wound, but the other comorbidities associated with thermal injuries. Pending the severity, treatments can include analgesics, oxygen supplementation, systemic antibiotics, and aggressive fluid therapy. Fluid loss from burn patients is a result of increased capillary permeability at the wound site and throughout the body in extensively burned patients9. This results in loss of plasma, electrolytes, and plasma proteins. This loss results in dramatic changes in cardiac output, hydration, and organ perfusion. This, in turn, causes shock, hypotension, and organ complications, such as acute kidney injury and non-specific hepatitis. Red blood cells also are damaged, which shortens life span and leads to secondary anemia. Patients often have the inability to retain heat due to the destruction of a large surface area of skin, along with heat loss from evaporation. This heat loss also is partly responsible for an increase in oxygen consumption and metabolic rate as the patient tries to regenerate heat. This causes a negative nitrogen balance, severe weight loss, and retards wound healing. The healing of burn wounds also is decreased when compared to other types of skin wounds due to tissue hypoxia in the wound and periwound region. This is due to the lack of perfusion from decreased cardiac output, hypovolemia, hyperviscosity, and vasoconstriction. This lack of perfusion leads to decreased delivery of healing cytokines, lymphocytes, neutrophils, and macrophages, which also leads to increased risk of infection and sepsis, and delays wound healing. Due to the nature of burn wounds and associated comorbidities, few injuries cause more damage to tissue and metabolism.
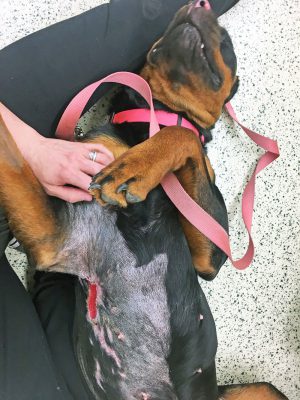
The presence of an inhalation injury significantly increases the morbidity and mortality associated with burn injuries. Smoke inhalation injury is attributed to inhaled toxic chemicals, and is a major cause of morbidity and mortality in thermally injured patients10. It causes injury to the trachea, bronchi, alveolar, and endothelial lining, and is responsible for the impairment of oxygen transport processes or oxygen utilization by inhalation of carbon monoxide and cyanide14. Inhalation injury immediately causes hypoxemia and mixed gas poisoning followed by tracheobronchitis, pneumonia, pulmonary atectasis, pulmonary embolisms, pulmonary congestion, and pulmonary edema due to direct injuries and secondary inflammatory reactions13. Heated air itself causes more upper airway injury and less lower airway pulmonary parenchyma injury like the inhaled toxic chemicals, such as the carbon monoxide10. This is due to mucosal fluid evaporation, heat dissipation by blood circulation, breath holding, and the air’s vortex motion10. During thermal, dry-air panting in dogs, there is a seven-fold increase in tracheal mucosal blood flow and about a three-fold increase in bronchial blood flow11. Temperature of the airway tissue increases immediately on the surface, while only slightly in the deeper tissues; heart and respiratory rates increase significantly12. If the air temperature is high enough to change the mucosal fluid into vapor, the local tissue would be damaged by the heat of the air directly and seriously9. This damages the glands, goblet cells ,and blood vessels, and causes marked swelling and edema. Decreased mucociliary transport impairs clearance of bacteria and mucosal debris14. Alveolar collapse and atelectasis can occur because of the loss of surfactant production or from the blockage of small airways from mucosal debris14. Treatment is aggressive supportive respiratory care with airway management. Intubation of burn-injured patients is not a benign intervention, as it can cause exacerbation of thermal laryngeal injury14. Surgery also can worsen anemia, hypotension, and hypothermia.
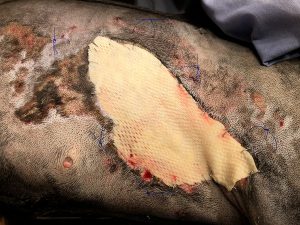
Burns are generally classified according to the depth of the burn and body surface area involved. There are two schemes for the classification of burn wounds. This includes degrees (1st through 5th) and partial- versus full-thickness. While the latter is more simplified, it recently has been deemed more appropriate for clinical applications, and there has been a shift into using this scheme. Partial-thickness burn wounds involve the epidermis and a portion of the dermis. Full-thickness burn wounds involve full-thickness loss of the epidermis and dermis. Burn depth is an important prognostic factor, as the loss of the dermal layer is the most common factor in serious burns and dictates most treatment. The assessment of the depth and surface area can be difficult in the initial phases due to fur coverage and time until the tissue declares itself as viable or nonviable. In Stella’s case, her wounds were difficult initially following arrival due to the above listed reasons and her lack of ability to handle manipulation outside of the oxygen-saturated kennel. Prior to her official consult 12 days following presentation, the MSU ECCM had placed Silver Sulfadiazine (SSD) cream, a broad spectrum water-miscible ointment, liberally on the obvious burn wounds until Stella’s wounds appeared to have declared themselves, and she was able to tolerate being out of the oxygen-rich kennel.
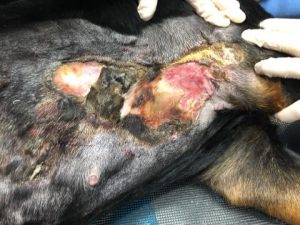
Full-thickness burn wounds are painful and slow healing. Due to destruction of tissues and associated nerves, the patient typically only has sensation to deep pressure. Tissue appears dry, hard, waxy, and white-to-gray or charred black in color. Loss of the epidermal barrier puts these patients at high risk for secondary infections, as burn tissue serves as a rich media for growth of bacteria. If the infection breaks through, then dissemination can occur. Systemic infection can be life-threatening. Septicemia is the most common cause for post-burn death15. Pseudomonas aeruginosa, staphylococcus, streptococcus, and micrococcus are common isolates from burn wounds15. Objectives of sepsis control involves proper cleansing, debridement of devitalized tissue, rapid epithelialization of the wound bed, early wound closure, topical antibiotics, and a clean environment15.
Typically, full-thickness wounds don’t heal quickly without surgical intervention and allowing the wound to heal by contraction; epithelialization with secondary wound healing leaves the wound open longer for continued fluid loss and infection. Early wound closure allows decreased evaporative loses and pain, increased phagocytosis of bacteria, stimulation of epithelial islands and granulation tissue, increased rate of healing, and decreased cost of associated hospitalization15. However, due to debilitation by the burn wound for the previously described reasons, primary wound closure or any autografts performed too soon after the injury are often slow in taking and still have delay in healing15. In addition, surgical options, such as any aggressive wound debridement, skin grafting, or flaps typically require general anesthesia to be performed.
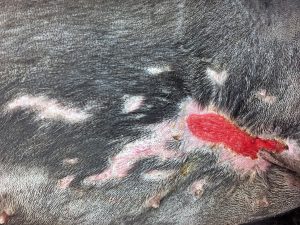
Upon examination of Stella’s large axillary wound, various options were discussed. Communication between surgery teams and the anesthesia and critical care teams is crucial to appropriately manage burn patients appropriately and effectively. Due to her severe smoke inhalation, secondary pneumonia, and oxygen supplementation requirement, she was considered too risky of an anesthesia candidate. As such, any surgical intervention left her at risk of furthering her respiratory injuries or disrupting her current cardiovascular stability. This left secondary wound healing with frequent bandage changes and repetitive debridement as the best option for Stella. Due to concerns for continued fluid loss and risk for infection, additional options were considered to help accelerate her healing. In recent years, there have been attempts to develop techniques and products to enhance the wound-healing processes. This includes quicker healing times and reduction in complications, infections, and death. These products allow for quicker cellular ingrowth, which is a fundamental first step to tissue regeneration6.
Tilapia skin dressings have been used in domesticated and wild animals in recent wildfires. This dressing was originally considered for Stella. The tilapia skin acts as a biological dressing. Tilapia skin dressings require complete debridement of the wound bed and placement of the tilapia dressing over the wound bed, while being secured with sutures or skin staples. The dressing remains in place while the patient scars naturally and then is removed after 7–14 days. For deeper wounds, it can be changed a few times throughout several weeks, if needed. During the healing process, the skin underneath is allowed to heal by second intention, and is not incorporated into the fish skin. These were originally studied in human medicine in Brazil. Due to no risk of zoonotic diseases between fish and canines, it does not require as aggressive of treatment of the skin in order to be used safely. Due to gentle processing, the structure and bioactive composition, including omega-3 polyunsaturated fatty acids, are preserved16. Studies have shown that omega-3 fatty acids possess antiviral17 and antibacterial18 properties, and also act as regulators of inflammation.19 But, it has not been FDA approved and is not commercially available. If used, the tilapia must be obtained from a market, skinned, and treated by the clinician. There isn’t a studied standardization; and proper handling of the fish prior to obtaining it cannot be confirmed.

Upon further investigation into more recent innovations within the field of wound healing, there was a fish-based product by Kerecis™ that was already FDA approved and commercially sold in the United States and Europe. It has already been extensively studied, and is currently being used in human medicine. It is an acellular dermal matrix (ADM). These nanofibrous wound dressings have numerous advantages in comparison to conventional dressings due to their high surface area and micro-porous and three-dimensional (3-D) structure2. They mimic the structure of the skin’s native extracellular matrix2, and quickly start signaling to attract fibroblasts to the dermal layer, which can excrete important extracellular matrix components to repair the damaged tissue2. ADM products are accepted as safe and effective in the treatment of hard-to-heal wounds of many etiologies in humans4,5. Mammalian ADM products possess prolonged shelf life when compared to cellular skin substitutes6. Currently used mammalian products include equine, bovine, and porcine. Concerns with the use of these mammalian-derived ADMs include the potential for autoimmune response, the potential risk of prion diseases, and cultural issues that may prohibit the use of porcine or bovine products20-21. Fish skin has been studied previously and was found to be remarkably similar to human skin when examined with electron microscopy3,6. In addition, the gentle processing as explained earlier retains the structure and bioactive composition including omega-3 polyunsaturated fatty acids,16 which possess antiviral,17 antibacterial,18 and anti-inflammatory properties.19
Kerecis™ Omega3 product is a full-thickness wild Icelandic cod meshed skin graft designed for use in burns, chronic skin wounds, hernia and dura repairs, and reinforcement of gastrointestinal-stapled incisions. Kerecis™ offers the advantage of an omega-3 fatty acid dressing and an acellular, non-mammalian matrix that neither induces an autoimmune reaction nor brings risk of communicable disease1. It is very porous due to the gentle treating, unlike other mammalian products; this allows for superior fibroblast colonization and limits Staphylococcus aureus growth up to 72 hours following placement, while also stimulating angiogenesis6-7. When compared to porcine submucosa ADM, the acellular fish skin graft resulted in faster healing time of cutaneous human skin defects8. It has been extensively studied for the safety, efficacy, and success in humans; however, until Stella, it had not been used in a veterinary setting. This was the first usage of Kerecis™ Omega3 BURN in a veterinary patient.

The graft is easily applied and doesn’t require specialized training. After grafting, the fish skin incorporates into the damaged area and is infiltrated by autologous cells that convert the graft into functional, living tissue while being slowly broken down6. The fish skin graft is reported to integrate into a wound bed in 7–10 days, based on previous human studies8. During that time, it provides a physical barrier that provides moisture retention to combat fluid losses and antimicrobial protection for 24–72 hours6-7.
Stella’s grafts were reapplied at times when the graft appeared to be completely incorporated with the naked eye. However, no biopsies were performed during her healing process to know if the graft incorporated in the same manner as those proven in human studies. She tolerated the placement of the grafts with minimal sedation, and only minimal debridement was performed during her wound care, so general anesthesia was never needed in Stella’s recovery. This allowed her larynx, trachea, and lungs to continue to heal throughout her recovery. While she developed recurring pneumonia throughout her initial three months post-incident and repeat radiographs showed significant fibrosis, she has been off of antibiotics for approximately two months and her respiratory signs have been improving. Her only reported clinical sign is slight exercise intolerance when compared to her housemate during intense play. There were no specialized instruments or technique needed in the placement of these grafts. The bandages that covered the graft were placed as directed by the Kerecis™ company in order to protect the moisture of the graft, as well as to absorb the exudate produced by the graft. Due to exudate production, her bandages were changed roughly every three–five days as needed, pending the amount of strikethrough on the bandage. She tolerated the bandage changes with minimal restraint. Within one month of her initial presentation, Stella no longer needed external bandages placed, and she was completely healed within three months. Throughout her recovery process, there were no obvious reactions to the use of the fish-based product; however, specific immunological testing was not performed to confirm this.
Limitations
As this was the first use of this product in a veterinary setting, there were obvious limitations encountered. No control population was available to help demonstrate if the graft truly increased the rate of her wound healing in comparison to traditional secondary wound healing techniques. No biopsies were performed throughout her wound healing or after graft placement to indicate how well the graft incorporated into the canine wound bed and how long it took to fully integrate. While extensive immunological testing has been performed in humans with this product to confirm its safety, there was no testing specifically with Stella to know there were no adverse effects that were potentially subclinical. As there were no previously described standard protocols in veterinary medicine, her treatments at each bandage change varied based on the state of her wound bed. In addition, this current product is cost prohibitive in the veterinary field. However, the Kerecis™ company is working on marketing a veterinary-specific product, which would be more affordable for clinical use.
Conclusion
While Stella’s case provided proof of a concept with this product in veterinary medicine, further investigations into using an acellular fish skin omega-3 rich graft should be undertaken in dogs with open wound healing. In this case, there were no complications or adverse effects encountered. Placement of this product doesn’t require anesthesia, and can be used in cases where there is concern for respiratory stability. Graft can be used for various sizes and shapes, and do not require specialized instruments or bandage materials.
References
- Magnusson, S., Kjartansson, H., Baldursson, B. T., Astradsdottir, K., Ågren, M. S., Hilmarsson, H., & Sigurjonsson, G. F. (2018). Acellular Fish Skin Grafts and Pig Urinary Bladder Matrix Assessed in the Collagen-Induced Arthritis Mouse Model. The International Journal of Lower Extremity Wounds, 17(4), 275–281.
- Gholipour-Kanani et a. (2018). Poly (ɛ-caprolactone)–chitosan–poly (vinyl alcohol) nanofibrous scaffolds for skin excisional and burn wounds in a canine model . IET Nanobiotechnology. 12 (5) 619-625
- Rakers S, Gebert M, Uppalapati S, et al (2010). “Fish matters”: the relevance of fish skin biology to investigative dermatology. Experimental Dermatology.19 (4): 313–24.
- Romanelli M, Dini V, Bertone MS. (2010). Randomized comparison of OASIS wound matrix versus moist wound dressing in the treatment of difficult-to-heal wounds of mixed arterial/venous etiology. Advanced Skin Wound Care. (23) 34-38
- Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D. (2005). OASIS Venus Ulcer Study Group. Effectiveness of an extra- cellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. Journal of Vascular Surgery. (41) 837-843.
- Lv Y-G, Liu J, Zhang J. (2006). Theoretical evaluation of burns to the human respiratory tract due to inhalation of hot gas in the early stage of fires. Burns. 32(4):436–46.
- Magnusson S, Baldursson BT, Kjartansson H, et al. (2015). Decellularized fish skin: characteristics that support tissue repair. Laeknabladid; 101(12) 567–73.
- Imai Y. (2015). Role of omega-3 PUFA-derived mediators, the protectins, in influenza virus infection. Biochim Biophys Acta. 1851(4) 496–502.
- Mil-Homens D, Bernardes N, Fialho AM. (2012). The antibacterial properties of docosahexaenoic omega-3 fatty acid against the cystic fibrosis multiresistant pathogen Burkholderia cenocepacia. FEMS Microbiol Lett. 328(1) 61–9.
- Serhan CN. (2014). Pro-resolving lipid mediators are leads for resolution physiology. Nature. 510 (7503) 92 – 101.
- Trautinger F, Kokoschka EM, Menzel EJ. (1991). Antibody formation against human collagen and C1q in response to a bovine collagen implant. Arch Dermatol Res. (283) 395-399.
- Charriere G, Bejot M, Schnitzler L, Ville G, Hartmann DJ. (1989). Reactions to a bovine collagen implant: clinical and immunologic study in 705 patients. Journal of the American Academy of Dermatology. (21) 1203-1208.
- Magnusson, Skuli & Baldursson, Baldur & Kjartansson, Hilmar & Rolfsson, Ottar & Fertram Sigurjonsson, Gudmundur. (2017). Regenerative and Antibacterial Properties of Acellular Fish Skin Grafts and Human Amnion/Chorion Membrane: Implications for Tissue Preservation in Combat Casualty Care. Military Medicine. 182. 383-388. 10.7205/MILMED-D-16-00142.
- Magnusson, Skuli & Baldursson, Baldur & Kjartansson, Hilmar & Ella Thorlacius, Guðný & Axelsson, Ivar & Rolfsson, Ottar & Petersen, Petur & Fertram Sigurjonsson, Gudmundur. Decellularized fish skin: characteristics that support tissue repair. Laeknabladid. 101. 567-573.
- Baldursson, B. T., Kjartansson, H., Konrádsdóttir, F., Gudnason, P., Sigurjonsson, G. F., & Helga Lund, S. (2015). Healing Rate and Autoimmune Safety of Full-Thickness Wounds Treated With Fish Skin Acellular Dermal Matrix Versus Porcine Small-Intestine Submucosa: A Noninferiority Study. The International Journal of Lower Extremity Wounds, 14 (1), 37–43.
- Pavletic, M. Michael, Trout J. Nicholas. (2006). Bullet, Bite, and Burn Wounds in Dogs and Cats. Veterinary Clinics of North America: Small Animal Practice. (36) 886-893.
- Wan, J., Zhang, G., Qiu, Y., Wen, C., Fu, T. (2016). Heat dissipation by blood circulation and airway heat absorption in a canine model of inhalation thermal injury. Burns. (42) 548-555.
- Baile, E.M., Guillemi, S., Pare, P.D. (1987) Tracheobronchial and upper airway blood flow in dogs during thermally induced panting. Journal of Applied Physiology. 63 (6) 2240-2246.
- Nie, F., Su, D., Shi, Y., Chen, J., Wang, H., Qin, W., Wang, S., Chen, Y. (2014). Early high volume lung lavage for acute severe smoke inhalation injury in dogs. Molecular Medicine reports. (9) 863-871.
- Bittner, E., Shank, E., Woodson, L., Martyn, J. (2015). Acute and perioperative care of the burn-injured patient. Anesthesiology. 122 (2) 448-464.
- Halbach, N., DeYoung, D. (1979). “Treatment and Management of major Burns,” Iowa State Univerisity. 41 (3) Article 5.